 Alkyl Halide Reactions Alkyl Halide Reactions
 The functional group of alkyl halides is a carbon-halogen bond, the common halogens being fluorine, chlorine, bromine and iodine. With the exception of iodine, these halogens have The functional group of alkyl halides is a carbon-halogen bond, the common halogens being fluorine, chlorine, bromine and iodine. With the exception of iodine, these halogens have 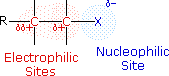 electronegativities significantly greater than carbon. electronegativities significantly greater than carbon.
 Consequently, this functional group is polarized so that the carbon is electrophilic and the halogen is nucleophilic, as shown in the drawing on the right. Consequently, this functional group is polarized so that the carbon is electrophilic and the halogen is nucleophilic, as shown in the drawing on the right.
 Two characteristics other than electronegativity have an important influence on the chemical behavior of these compounds. The first of these is covalent bond strength. The strongest of the carbon-halogen covalent bonds is that to fluorine. Two characteristics other than electronegativity have an important influence on the chemical behavior of these compounds. The first of these is covalent bond strength. The strongest of the carbon-halogen covalent bonds is that to fluorine.
 Remarkably, this is the strongest common single bond to carbon, being roughly 30 kcal/mole stronger than a carbon-carbon bond and about 15 kcal/mole stronger than a carbon-hydrogen bond. Because of this, alkyl fluorides and fluorocarbons in general are chemically and thermodynamically quite stable, and do not share any of the reactivity patterns shown by the other alkyl halides. Remarkably, this is the strongest common single bond to carbon, being roughly 30 kcal/mole stronger than a carbon-carbon bond and about 15 kcal/mole stronger than a carbon-hydrogen bond. Because of this, alkyl fluorides and fluorocarbons in general are chemically and thermodynamically quite stable, and do not share any of the reactivity patterns shown by the other alkyl halides.
 The carbon-chlorine covalent bond is slightly weaker than a carbon-carbon bond, and the bonds to the other halogens are weaker still, the bond to iodine being about 33% weaker. The carbon-chlorine covalent bond is slightly weaker than a carbon-carbon bond, and the bonds to the other halogens are weaker still, the bond to iodine being about 33% weaker.
 The second factor to be considered is the relative stability of the corresponding halide anions, which is likely the form in which these electronegative atoms will be replaced. The second factor to be considered is the relative stability of the corresponding halide anions, which is likely the form in which these electronegative atoms will be replaced.
 This stability may be estimated from the relative acidities of the H-X acids, assuming that the strongest acid releases the most stable conjugate base (halide anion). With the exception of HF (pKa = 3.2), all the hydrohalic acids are very strong, small differences being in the direction HCl < HBr < HI. This stability may be estimated from the relative acidities of the H-X acids, assuming that the strongest acid releases the most stable conjugate base (halide anion). With the exception of HF (pKa = 3.2), all the hydrohalic acids are very strong, small differences being in the direction HCl < HBr < HI.
|